
 |
|
|
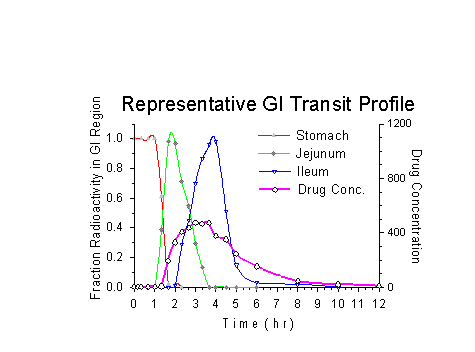
Formulation development success depends on defining the variables that affect the performance of a drug delivery system. Often, in vitro testing methods are not predictive of in vivo results. For oral dosage forms, altered gastrointestinal transit due to individual variation, physiologic or pharmacologic factors, or the presence of food, may influence bio-availability. Disintegration, erosion, or drug release may be premature or delayed in vivo. Similarly, altered deposition or clearance from other routes of administration such as nasal, ocular, or inhalation may explain drug absorption anomalies. Gamma scintigraphy combined with knowledge of physiology and dosage form design can help define these variables. The resulting insight can be used to accelerate the formulation development process and help ensure success in early clinical trials. Products with little chance of attaining their goals can be abandoned earlier. The performance of viable products can be clearly demonstrated to regulatory authorities or potential investors. |
|
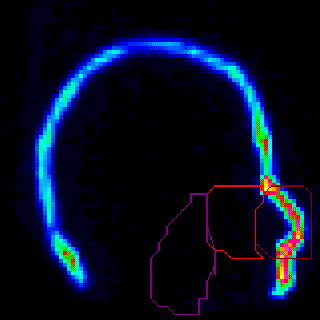
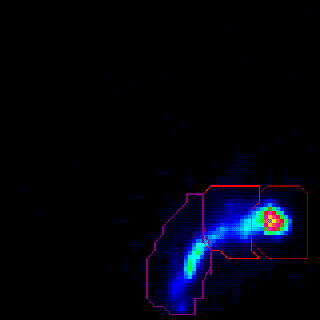
Nasal Deposition and Clearance |
 |
|||
 |
|||
|
Standard radiolabeling techniques incorporate the radioactive marker in a finished product shortly before dose administration. Alternatively, neutron activation is a technique where a small amount of stable isotope is incorporated in the dosage form at the time of manufacture. This allows the product to be produced in the sponsor's facility under normal manufacturing conditions. The stable isotope is then converted to a radioactive isotope appropriate for gamma scintigraphy by a short exposure to a neutron flux. |
|||
 |
|
Human clinical studies and data analysis are performed in Scintipharma's private facility with appropriate regulatory approvals. In-house overnight lodging of research study subjects is available to ensure protocol compliance. Contract cGMP manufacturing sites are convenient within the local area for production of small scale batches of pharmaceutical formulations for these gamma scintigraphy studies. Animal studies are performed in collaboration with the pharmaceutical science graduate program at a nearby academic institution of higher education which is well known for excellence. |
|
[Home] |